Progression-free survival at final analysis
ZYDELIG + R median PFS 19.4 months vs 6.5 months with rituximab alone1
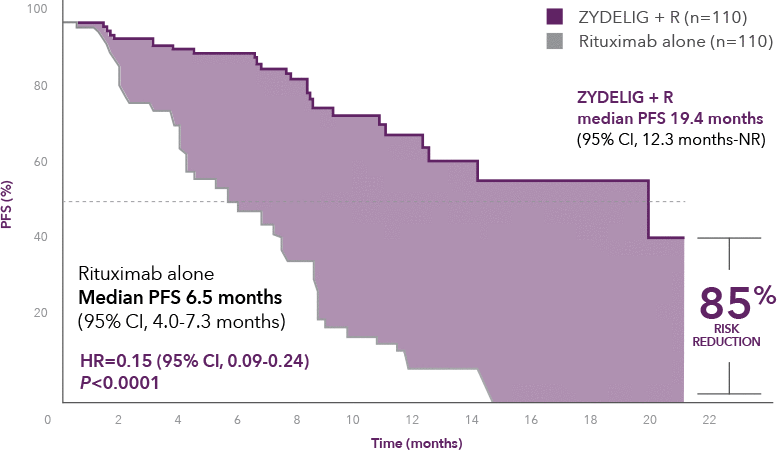
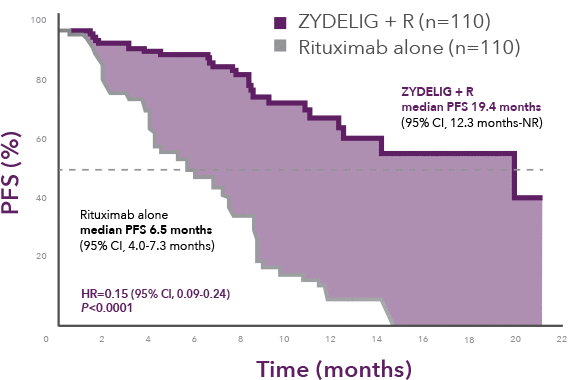
66% of patients were progression-free at 1 year on ZYDELIG + R vs 13% with rituximab alone8
Pinch to zoom
ZYDELIG + R provides consistent results across prespecified patient subpopulations

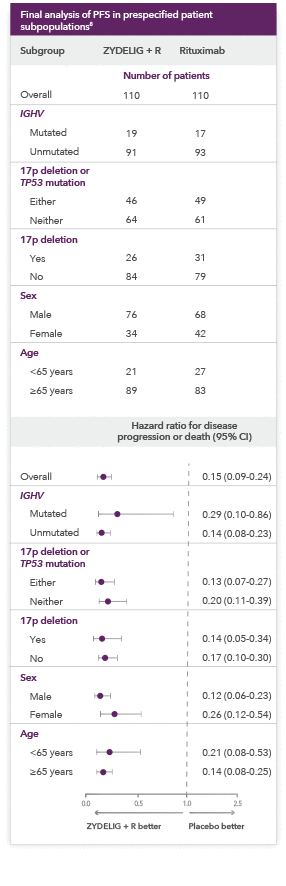
-
Hazard ratios of less than 1.00 for disease progression or death indicate better results in the ZYDELIG + R arm
Important Limitations: Data from the subgroup analysis of PFS are not included in the ZYDELIG Prescribing Information. Caution should be used when interpreting the results of the subgroup analysis due to the number of patients.
Pinch to zoom